Cheminformatics Tutorials - Herong's Tutorial Examples - v2.03, by Herong Yang
Ring Represenations in SMILES
This section provides a quick introduction on ring represenations in SMILES. Append a numeric digit to the starting atom of a ring and close it with the numeric digit as the start atom.
How Rings Are Represented in SMILES? - Rings are represented in SMILES according to the following rules:
1. Append a numeric digit to the starting atom of a ring as its identifier. For example, "C6..." starts ring "6" at atom C.
2. Use the numeric digit associated to the ring to represent the starting atom to the closing bond to the last atom. For example, "...=6" closes ring "6" with a double bond.
Note that closing bond symbol can be omitted, if it is a single bond, or an aromatic bond with atoms in lower letters. For example, C1CCCCC1 is the same as C1CCCCC-1; c1ccccc1 is the same as c1ccccc:1.
3. A ring identifier can be re-used, if the identified ring is already closed. For example, O1CCCCC1N1CCCCC1 is the same as O1CCCCC1N2CCCCC2.
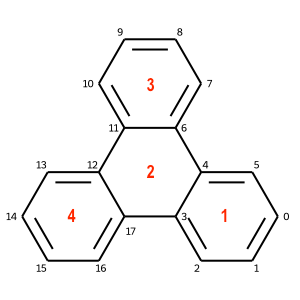
Note that rings can be nested represent multiple connected rings. For example, 'c1ccc2c(c1)c3ccccc3c4ccccc42' represents 4 connected rings as shown below:
4. If single numerical digits are not enough to identify rings, double digits can used with a prefix of "%" symbol. For example, C60 molecule can represented in SMILES as:
C12=C3C4=C5C6=C1C7=C8C9=C1C%10=C%11C(=C29)C3=C2C3=C4C4=C5C5=C9C6=C7C6=C7C8 =C1C1=C8C%10=C%10C%11=C2C2=C3C3=C4C4=C5C5=C%11C%12=C(C6=C95)C7=C1C1=C%12C5 =C%11C4=C3C3=C5C(=C81)C%10=C23
Table of Contents
►SMILES (Simplified Molecular-Input Line-Entry System)
Branch Represenations in SMILES
►Ring Represenations in SMILES
Disconnected Structures in SMILES
Charge Represenations in SMILES
Isotope Represenations in SMILES
Chirality Representations in SMILES
Hydrogen Representations in SMILES
Open Babel: The Open Source Chemistry Toolbox
Using Open Babel Command: "obabel"
Generating SVG Pictures with Open Babel
Substructure Search with Open Babel
Similarity Search with Open Babel
Fingerprint Index for Fastsearch with Open Babel
Stereochemistry with Open Babel
Command Line Tools Provided by Open Babel
RDKit: Open-Source Cheminformatics Software
rdkit.Chem.rdchem - The Core Module
rdkit.Chem.rdmolfiles - Molecular File Module
rdkit.Chem.rdDepictor - Compute 2D Coordinates
rdkit.Chem.Draw - Handle Molecule Images
Molecule Substructure Search with RDKit
rdkit.Chem.rdmolops - Molecule Operations
Daylight Fingerprint Generator in RDKit
Morgan Fingerprint Generator in RDKit
RDKit Performance on Substructure Search
Introduction to Molecular Fingerprints
OCSR (Optical Chemical Structure Recognition)
AlphaFold - Protein Structure Prediction