Cheminformatics Tutorials - Herong's Tutorial Examples - v2.03, by Herong Yang
rdkit.Chem.rdSubstructLibrary - Substructure Library
This section provides a quick introduction on rdkit.Chem.rdSubstructLibrary module in RDKit library that provides functionalities to do substructure search with a molecule library.
What Is rdkit.Chem.rdSubstructLibrary Module? rdkit.Chem.rdSubstructLibrary module in RDKit library provides functionalities to do substructure search with a molecule library.
rdkit.Chem.rdSubstructLibrary module contains one main class: rdkit.Chem.rdSubstructLibrary.SubstructLibrary, which has the following main methods:
l = rdSubstructLibrary.SubstructLibrary() - Creates a SubstructLibrary object.
i = 1.AddMol(m) - Adds the given molecule to the library and returns the index of the added molecule.
c = 1.CountMatches(s) - Performs substructure match in the library and returns the number of matched molecules.
i = 1.GetMatches(s) - Performs substructure match in the library and returns a list of matched molecule indexes. The default number of maximum matched molecule indexes is 1000.
m = 1.GetMol(i) - Returns the molecule of a given index.
Here is a short Jupyter Notebook example of using rdkit.Chem.rdSubstructLibrary.SubstructLibrary class for substructure search.
from rdkit import Chem
ms = ["O=C(O)c2cc1ccc(Cl)n1cn2", "O=C(O)c2ccn1c(Cl)ccc1n2"]
ms = list(map(Chem.MolFromSmiles, ms))
i = Chem.Draw.MolsToGridImage(ms)
display(i)
s = Chem.MolFromSmiles('c1nccc2n1ccc2')
i = Chem.Draw.MolToImage(s, size=(120,120))
display("Substructure", i)
from rdkit.Chem import rdSubstructLibrary
l = rdSubstructLibrary.SubstructLibrary()
for m in ms:
l.AddMol(m)
c = l.CountMatches(s)
print("Number of matches", c)
ms = l.GetMatches(s)
ms = [l.GetMol(m) for m in ms]
i = Chem.Draw.MolsToGridImage(ms)
display(i)
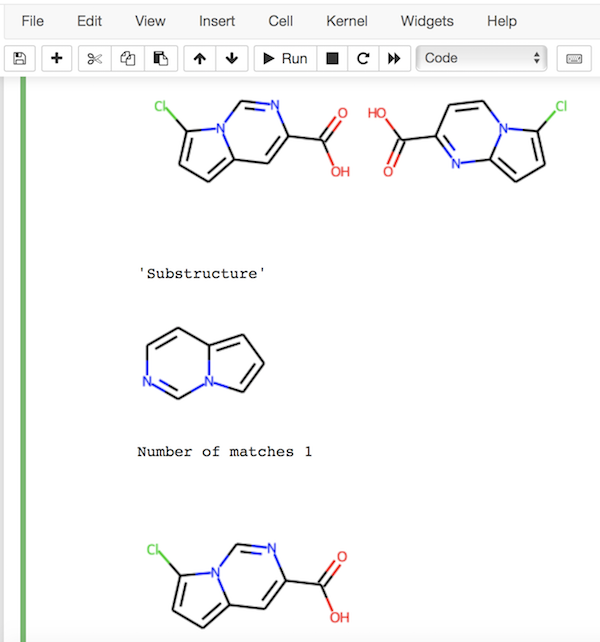
Table of Contents
SMILES (Simplified Molecular-Input Line-Entry System)
Open Babel: The Open Source Chemistry Toolbox
Using Open Babel Command: "obabel"
Generating SVG Pictures with Open Babel
Substructure Search with Open Babel
Similarity Search with Open Babel
Fingerprint Index for Fastsearch with Open Babel
Stereochemistry with Open Babel
Command Line Tools Provided by Open Babel
RDKit: Open-Source Cheminformatics Software
rdkit.Chem.rdchem - The Core Module
rdkit.Chem.rdmolfiles - Molecular File Module
rdkit.Chem.rdDepictor - Compute 2D Coordinates
rdkit.Chem.Draw - Handle Molecule Images
►Molecule Substructure Search with RDKit
RDKit m.HasSubstructMatch(s) - Substructure Match
RDKit GenerateDepictionMatching2DStructure(m, s) - Substructure Orientation
RDKit rdMolDraw2D.PrepareAndDrawMolecule - Substructure Highlight
RDKit Substructure Search with SMARTS
rdkit.Chem.rdFMCS - Maximum Common Substructure
►rdkit.Chem.rdSubstructLibrary - Substructure Library
Substructure Library in Binary and SMILES Formats
rdkit.Chem.rdmolops - Molecule Operations
Daylight Fingerprint Generator in RDKit
Morgan Fingerprint Generator in RDKit
RDKit Performance on Substructure Search
Introduction to Molecular Fingerprints
OCSR (Optical Chemical Structure Recognition)
AlphaFold - Protein Structure Prediction